Biotron is proud to collaborate with Eureka Lab Division, a company renowned for its expertise in developing advanced analytical methods, particularly for the determination of urinary and plasma catecholamines using HPLC with fluorimetric detection—critical in supporting accurate cancer diagnosis in collaboration with leading oncologists.
Many years ago, Dr. Gilberto Coppa , Dr. Stefano Sartori and dr. Angela Cusenza decided to pursue a professional path that few companies had dealt with:
Simplify, Improve, Standardize
the analytical procedures existing in clinical laboratories, thanks to the realization of Ready to use Kits using HPLC, GC, GC-MS, LC-MS/MS chromatographic techniques.
From the very outset, they concentrated their activity on the development of an Analytical Method for the Determination of Urinary and Plasma Catecholamines in HPLC with Fluorimetric Detection.
The decision to work with Catecholamines was due to the shared thought, along with many Oncologists, that the need of Formulating a Correct Diagnosis of cancer patients was fundamental and extremely important
The concentration of catecholamines (Noradrenaline, Adrenaline, Dopamine), which are a group of hormones produced by the adrenal glands, are greatly increased in biological fluids in the presence of such pathologies.
To help doctors to properly diagnose these tumor forms, they asked some laboratories about their problems and issues in their structures since the 1970s.
Here are some of the problems raised:
After collecting these information relative to the problems arising from the use of the amperometer and coulometric detectors they decided to “create”
Our team worked long for the development of a fluorimetric, chromatographic, analytical method for the determination of catecholamines with the benefit that:
Their work involved the development of a biologic matrix purification method that was more self-explanatory than the methods used up to that time.
After purification, the sample undergoes a process of derivatization.
At this point, the derivatized sample is read in fluorimetry, allowing it to obtain excellent sensitivity and specificity for the analysis.
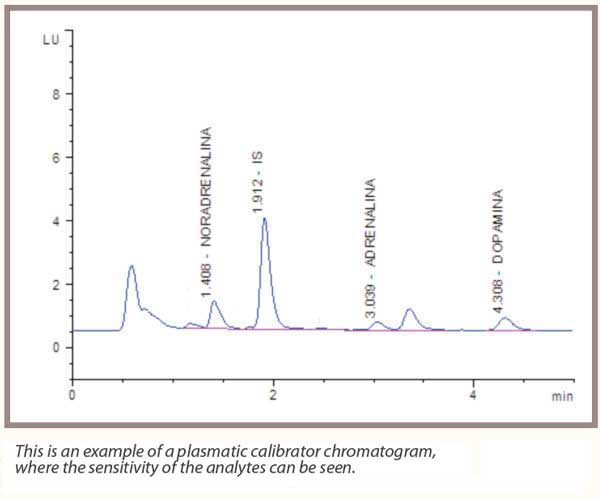
The analytical procedures existing in clinical laboratories, regarding the dosage of both urine and plasma catecholamines.
But this was just the beginning … Since 2000 the Eureka Lab Division Team, has continued to develop
Initially the Team dealt with
Liquid Chromatography (HPLC / UHPLC) coupled to detectors:
They then dedicated ourselves to
Gas Chromatography (GC) coupled to detectors:
Finally, since 2008, we have focused our work on
Liquid Chromatography (LC) coupled with
Mass Spectrometer / Triple Quadruple / Ion Trap
In order to be able to continuously increase the range of new Eureka Kits,
the company has for several years been working with the
They are Specialists in Clinical Biochemistry, Pharmaco – Toxicology and expert in
Chromatographic Analytical Techniques.
They collect the requests of Doctors who are in close contact with patients and iniziate for the development of Chromatographic Analytical Methods that will then be engineered into EUREKA KITS.
It’s like having many research and development teams across Italy working
to increase the development of new chromatographic analytical methods
that will then be engineered in the form of ready-to-use kits.
After a long research and development work by the Eureka Lab Division Team and by the Study Group of External Authors, Eureka Kit are Validated to GUARANTEE their
©2024 biotron.co.il All Rights Reserve | Built by HDigital | הצהרת נגישות
מופעל על ידי אבירם אזיאל | led by CEO Aviram Aziel